Archive
LORIER webinar about FAIRification of Clinical Trial Data, on January 9, 2026, at 12:30 p.m.
2026.01.06
Ka Hin Tai will present a comprehensive and practice-oriented framework for the FAIRification of biomedical research data. Rooted in the Findable, Accessible, Interoperable, and Reusable principles, the session will walk through key steps to plan prospectively for data sharing, prepare datasets and metadata that support meaningful reuse, and manage data-hosting, governance processes, and long-term stewardship after upload. For more information please visit https://restores.univ-rennes.fr/highlights/lorier-webinar-series-reproducibility-and-meta-research.
Professional Traininig 2 - Career opportunities in industry and academia
2025.12.10
On December 4th, 2025, our doctoral students attended an inspiring professional training workshop hosted by Prof. Christoph Gerlinger (Bayer AG) & Martina Esdaile (ECRIN), featuring two excellent speakers who shared open and insightful perspectives on career paths in industry and academia.
-
Dr. Katrin Walkamp, Lead Statistician at Bayer AG, walked us through her journey from Biology and Mathematics into clinical statistics. She explained the organizational structure at Bayer, the three stages of clinical trials in drug development, and offered a behind-the-scenes look at the work environment, interdisciplinary team dynamics, the responsibilities of a project statistician, and broader data science opportunities beyond traditional biostatistics.
-
Dr. Darren Dahly, Principal Statistician at the HRB Clinical Research Facility, University College Cork, shared a reflection on his nonlinear academic career. He spoke about embracing uncertainty, following unexpected opportunities, and the importance of flexibility, applied methods, and essential “soft” skills such as writing, communication, collaboration, and leadership.
Our doctoral researchers also had the chance to ask questions, engage with the speakers, and reflect on their own career paths. The insights and conversations shared during the session sparked fresh motivation and inspiration, encouraging them to explore different possibilities for their professional development.
We extend our sincere thanks to our speakers for sharing their experiences, and to our students for their curiosity and active engagement!
Presentation at ASA Biopharmaceutical Section’s 38th Virtual Discussion
2025.12.09
Martin Posch (MUW) presented work on Han Chang Chiam's first PhD manuscript on hybrid RCTs today. It was for the American Statistical Association Biopharmaceutical Section's 38th Virtual Discussion on "Statistical Considerations for Hybrid Control Arms in Cancer Clinical Trials." The FDA Oncology Center of Excellence also plans to publish a summary of the meeting in the Biopharmaceutical Report.
Workshop: Scientific Writing
2025.12.03
As part of our ongoing professional training programme, we organised a two-day workshop on Scientific Writing, led by Tim Korver. The workshop focused on providing our doctoral students with clear, practical tools to strengthen their scientific communication.
Over the two afternoons (2025.11.27 & 2025.12.01), our researchers:
• Learned fundamental principles of clarity, cohesion, and coherence
• Practiced applying these principles to real scientific texts
• Mapped key messages using structured frameworks
• Completed exercises on sentence flow, topic–stress positioning, and improving paragraph cohesion
• Analysed examples of good scientific writing
• Practiced revising their own work and supporting peers
• Learned about the Scientific Storytelling Arc and how to use it to structure their research narratives
• Reflected on ways AI tools can support the writing process
• Shared practical insights and experiences to enhance writing productivity
• Completed a 20-minute focused writing session, followed by peer feedback exchanges to improve clarity, structure, and communication
The workshop included targeted activities and example texts designed to help researchers strengthen how they present problems, structure arguments, and create flow across paragraphs. This interactive training forms an important part of the SHARE-CTD commitment to equipping our doctoral candidates with strong research communication skills.
A warm thank-you to Tim Korver for the excellent training and to all our doctoral researchers for their active participation.
Professional Traininig 2 - Data access & communication between stakeholders
2025.11.11
This professional training brought together leading experts from academia, industry, and research infrastructures to discuss patient-level data access and the future of data sharing in health research.
The session began with a comprehensive overview of the Vivli data sharing platform, presented by Julie Wood (COO, Vivli), Catrin Tudur Smith (Professor of Biostatistics, University of Liverpool), Rebecca Sudlow (Global Lead Patient Level Data Sharing, Roche), David McAllister (Professor of Clinical Epidemiology and Medical Informatics, University of Glasgow), and Georgina Humphreys (Secretariet Independent Review Panel). The speakers explained how researchers can access patient-level data for evidence synthesis, the difference between individual patient data (IPD) and summary-level data, and the key steps from data request to analysis and dissemination. Case studies illustrated the workflow from both the researcher’s and data provider’s perspectives, highlighting Vivli’s transparent and collaborative approach to responsible data reuse.
Following this, Catia Sousa Pinto (Shared Services for Ministry of Health, EPE) presented the European Health Data Space (EHDS), which is a landmark EU initiative establishing common standards, governance, and infrastructure to facilitate secure, cross-border health data sharing. The EHDS aims to empower citizens, support evidence-based policymaking, and advance research and innovation across Europe.
Finally, Sergio Contrino (Head of Data Projects, ECRIN) introduced tools developed by ECRIN, including the Metadata Repository and Data Sharing Repository, which promote interoperability and sustainable access to clinical research data within the broader European Open Science Cloud (EOSC) ecosystem.
This training offered valuable insights into how collaborative infrastructures like Vivli, EHDS, and ECRIN are shaping the future of open and responsible data sharing in health research.
Professional Orientation
2025.10.30
On November 6, 2025, and December 4, 2025, the fifth training event for our 11 doctoral students will take place online. The topics of the training are “Data Access and Communication between Stakeholders” and “Career Opportunities in Industry and Science” and will be presented by guest speakers from Bayer AG and ECRIN.
Second Schooling in Lyon
2025.10.25
From 20 to 24 October 2025, the 2nd Schooling took place in person at the Domaine Lyon Saint Joseph in Lyon, bringing together international doctoral students, principal investigators, partners and guest speakers.

Topics such as IPD-MA, data protection, meta-research, the GDPR, and the legal aspects of data sharing were on the agenda, as were panel discussions with two patients representants as well as a discussion with a sociologist about marginalised populations.
Alongside the demanding and intensive scientific programme, there was also time for culinary and cultural experiences, including a guided tour of Lyon's old town, visits to the Musée Lumière, and a meal at a traditional Lyonnais restaurant, which provided a welcome break.

We will meet again during the week beginning 8 February 2026 in Zeist, the Netherlands, where Datathon 2 will take place under the direction of Valentijn de Jong (University Medical Centre Utrecht). Then it will be time for the doctoral students to get back to programming, analysing and presenting!
Professional Training 2: Presentation Skills
2025.10.20
On October 19, 2025, as part of our professional training, our doctoral students came together in Lyon for a full-day Presentation Skills workshop led by S. Lerch. This hands-on training focused on turning complex research into clear, compelling talks. Every doctoral student had an opportunity to deliver a short presentation and receive detailed feedback on structure, delivery, body language, and slide design. They finished the day with an interactive session on nervousness and stage fright, exploring the underlying causes and learning practical strategies to stay calm and confident on stage.
Huge thanks to S. Lerch for the energetic coaching and structured feedback, and to our doctoral students who leaned in, practiced, and gave thoughtful peer reviews.
We’re walking away with personal presentation profiles and a clear plan to keep improving every talk we give.
SHARE-CTD doctoral student submitted manuscript to Statistics in Medicine
2025.10.07
Han Chang Chiam (MUW) has submitted a manuscript to Statistics in Medicine. The arXiv preprint is available here: https://arxiv.org/abs/2510.04829.
Professional Training 1: Team Building & Management
2025.10.07
Last week, our doctoral students participated in an engaging Team Building and Management Workshop, led by S. Lerch, aimed at enhancing collaboration, communication, and leadership across our international consortium.
The workshop covered both practical and theoretical topics, including effective delegation, time management, problem-solving, and conflict resolution. Through interactive exercises and group discussions, participants explored key principles of effective teamwork, such as recognizing diverse personalities, defining project leadership roles, and maintaining team cohesion and motivation.
The session fostered a supportive environment that encouraged self-reflection, creativity, and shared responsibility, which are essential elements for a thriving interdisciplinary research setting. Doctoral students also had the opportunity to reflect on their team dynamics, discuss ground rules, and share expectations. This interactive experience offered valuable insights into trust-building, cultivating a feedback culture, and navigating conflict, ultimately supporting stronger coordination and collaboration within the team.
Primer: Conducting an Individual Participant Data Meta-Analysis
2025.10.01
Gorka Fraga Gonzalez, Leonhard Held and Fabio Molo (UZH) wrote a primer on conducting individual patient data meta-analyses. The primer can be downloaded via Zenodo (https://zenodo.org/records/17153077).
SHARE-CTD doctoral student published her first paper in JCE
2025.09.22
Giulia Varvarà (UNIVREN) has published her first paper “Reusing clinical trial data to consolidate and advance medical knowledge" in the Journal of Clinical Epidemiology (JCE) with Clara Locher, Cora Burgwinkel, Ulrich Mansmann, Florian Naudet and Joseph Ross. The abstract can be read here: https://pubmed.ncbi.nlm.nih.gov/40976520/. Congratulations!
SHARE-CTD doctoral student published his first paper in JCE
2025.09.20
Ka Hin Tai (UNIVREN) has published his first paper in the Journal of Clinical Epidemiology (JCE) "Key concepts in clinical epidemiology: FAIRification of biomedical research data.", together with Ulrich Mansmann, Evelyne Decullier, Florian Naudet, Fabian Prasser, Ulrich Sax, and others. The full article can be read here: https://www.jclinepi.com/article/S0895-4356(25)00253-7/fulltext. Congratulations!
SHARE-CTD doctoral students participate in ROeS and ISCB 2025
2025.09.19
Cora Burgwinkel (UZH) and Han Chang Chiam (MUW) participated in ROeS (https://www.roes2025.at/) in September 2025 and successfully presented their PhD projects: Cora Burgwinkel gave a presentation on “Adjusting for Outcome Reporting Bias: A Multiple Imputation Approach,” while Han Chang Chiam spoke on “Selection Bias in Hybrid Randomized Controlled Trials Using External Controls". Cora Burgwinkel also participated in ISCB 2025 (https://iscb2025.info/) and presented her project. Congratulations to both of them! Further information is also available on our LinkedIn account.
Second Datathon in February 2026 in Utrecht
2025.09.09
Under the lead of PhD Assistant Professor Valentijn de Jong (University Medical Center Utrecht) the second SHARE-CTD Datathon will take place in Zeist from February 9 to 13, 2026.
LORIER Webinar Series on Reproducibility and Meta-Research
2025.08.29
The second LORIER webinar will take place on Friday, September 5, at 12:30 p.m. It is entitled “Training the next generation of researchers in the field of clinical data exchange” and will be presented by Ulrich Mansmann and moderated by Florian Naudet. For more information, please visit the following website: https://restores.univ-rennes.fr/highlights/lorier-webinar-series-reproducibility-and-meta-research.
"Scientific Writing"
2025.08.13
On November 27, 2025, and December 1, 2025, the fourth professional training session for our 11 doctoral students will take place online. The topic of the training is Scientific Writing and will be conducted by T. Korver.
"Presentation Skills"
2025.07.29
On 19.10.2025 the third full-day professional training of our doctoral students will take place in person in Lyon under the direction of Sabine Lerch. The topic will be presentation skills.
"Team Building & Management"
2025.07.29
On 02.10.2025 the second full-day professional training of our doctoral students will take place virtually under the direction of Sabine Lerch. The topics will be team building and management.
Professional Training 1: “Managing Stress & Interacting in a Multicultural Setting”
2025.07.18
On July 17, 2025, as part of the SHARE-CTD professional training program, all doctoral students participated in an interactive full-day workshop focused on managing stress and interacting in a multicultural setting, facilitated by Sabine Lerch. The workshop provided valuable tools for recognizing and managing stress, as well as recognizing cultural differences and improving communication in diverse academic and professional environments.
The training covered a range of topics, including identifying different types and sources of stress, strategies to manage the stress cycle, understanding cultural differences and typical conflicts in multicultural environments and practical skills for more effective intercultural interactions.
Through interactive exercises, reflection activities, and group discussions, participants were encouraged to explore their own stressors and cultural backgrounds while building strategies for well-being and effective collaboration in international settings.
The workshop was highly appreciated for its practical relevance and inclusive atmosphere. It provided doctoral students with essential tools to improve both their well-being and effectiveness in multicultural environments. This training was part of the SHARE-CTD network’s broader effort to equip early-stage researchers with not only academic skills but also the personal and interpersonal competencies necessary for success in global research collaborations.
First Datathon in Göttingen
2025.07.14
From 07.07. to 11.07.2025, the 1st Datathon took place in person at the Rechenzentrum Göttingen with international doctoral students, principal investigators and guest lecturer as part of the SHARE-CTD project.

In addition to guest lectures on a case study, lectures and exercises on clinical trial, reproducibility, biostatistics, data management and reporting guidelines, programming, presentations and discussions in five groups made up the agenda.

Winner of Datathon 1
As a balance to the tight and demanding program, all participants were able to enjoy a “rainy” barbecue, an exciting city tour and delicious German and Italian dishes in the evening. And what would a 1st Datathon be without a second one? This will take place in Utrecht at the beginning of 2026 under the lead of Valentijn de Jong. But before we go there, we will meet again in October 2025 for the 2nd Schooling in Lyon, where the doctoral students will be prepared for Datathon 2.

Responsible Research in Action Unconference, Berlin, September 2025
2025.07.01
Registration for the Responsible Research in Action unconference (Berlin, Sept 22-24) is now open: https://rr-in-action2025.org/registration-and-presentation-of-the-available-projects https://rr-in-action2025.org/registration-and-presentation-of-the-available-projects /. Participants will work on one of ten project to improve research culture and practice - topics range from research assessment to reproducibility to accessibility of informed consent forms to research culture. On the registration site, you'll find a short video explaining each project. If you would like to attend, it's important to register quickly, as there are only 15-20 spaces per project. There will be pre-conference workshops on the 22nd before the unconference, and the conference dinner will be held in the dinosaur room of the Berlin natural history museum.
Mid-term check meeting in June 2025
2025.06.26
On 18.06.2025 our full day mid-term check meeting with all our PIs and DCs as well as some partners and the Project Officer at REA took place online. Today we received her feedback and recommendations and our project appears to be fully on track. Thank you all for your active participation!
"Managing Stress & Interacting in a Multicultural Setting"
2025.06.25
On 17.07.2025 the first full-day professional training of our doctoral students will take place virtually under the direction of Sabine Lerch. The topics will be managing stress and interacting in a multicultural setting.
Presentation on "Clinical Trial Data Sharing: Uses and Challenges in Implemention" by Martin Posch
2025.06.25
Martin Posch gave a (virtual) lecture yesterday at the University of Würzburg as part of the IKE-B & ImDS institute seminar on “Clinical Trial Data Sharing". 2025_06_Wuerzburg_Data_Sharing 2.pdf
"OSIRIS Items for Reproducibility & Invitation to Join Interventional Studies" - LORIER webinar
2025.06.10
The first LORIER webinar willl take place on Friday, June 13th, at 12:30. For more information, please visit the following website: https://restores.univ-rennes.fr/our-events/osiris-items-for-reproducibility-and-invitation-to-join-interventional-studies-by-rita-banzi?oac=eyJpbmRleCI6MCwidG90YWwiOjMsImZpbHRlcnMiOnsiZGV0YWlsZWQiOjF9fQ%3D%3D
New article published by the team of Evgeny Bobrov at the BIH
2025.06.03
A team around Evgeny Bobrov at the BIH (including Tracey Weissgerber) published an article on „Six Solutions for Clinical Study Data Sharing in Germany“ (https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874-025-02560-y). The paper adresses obstacles to trial data sharing in Germany and propose six solutions targeting ethics committees, trial registries, infrastructures, and governance. The paper also contains input from other SHARE-CTD colleagues.
The French government is addressing the issue of clinical trial transparency
2025.05.20
Two French ministries (Research and Health) have signed recently an important new policy document: https://www.enseignementsup-recherche.gouv.fr/sites/default/files/2025-05/clinical-trials-report---en-36812.pdf . This initiative is part of the broader national strategy on open science: https://www.ouvrirlascience.fr/national-plan-for-open-science-4th-july-2018/. The report focuses on results posting, not data sharing per se, but the topics are closely related and highly relevant to our work. Notably, one of the key recommendations (1.10) is: "Continue the work on improving clinical trial transparency by creating a working group on clinical trials data sharing. On several occasions, the Working Group has discussed the need to put forward proposals of guidelines for data and documentation sharing in clinical trials. Given the complexity of the task, the group suggests initiating a new, dedicated project once this group’s recommendations on trial results posting have been published."
Revision of the SPIRIT and CONSORT guidelines
2025.05.20
SHARE-CTD welcomes the important 2025 revision of the SPIRIT and CONSORT guidelines: https://www.consort-spirit.org/. Both helpauthors to design and report their trials completely and transparently. The 2025 versions provides a new section on open science addressing issues of accessibility of trial protocol and statistical analysis plan (Item 5) and data sharing (Item 6). The new item lay the foundation for a FAIR sharing of clinical trial data (SHARE-CTD): Where and how the individual de-identified participant data (including data dictionary), statistical code, and any other materials will be accessible. This is an important opportunity to raise awareness that evidence created from this data can be used by regulatory bodies, health technology assessment agencies, and clinical medicine to the provide more profit to patients.
LORIER Webinar Series on Reproducibility and Meta-Research
2025.05.06
LORIER launches a series of webinars on reproducibility and meta-research The LORIER Ambassadors Program for Reproducibility and Meta-Research kicks off a series of monthly webinars dedicated to the challenges of reproducible health research. Organized in partnership with OSIRIS, SHARE-CTD, Restores, and the French Network for Reproducible Research, this series will feature international speakers and a variety of topics: sharing clinical data, tools for reproducibility, ambassador networks, and FAIRification of data. The dates and agenda are published via https://restores.univ-rennes.fr/highlights/lorier-webinar-series-reproducibility-and-meta-research.
New article "The validation of prediction models deserves more recognition" published in BMC Medicine
2025.03.24
Begüm Irmak Ön and Ulrich Mansmann published a comment paper on the scarcity of external validations and impact studies of clinical prediction methods hinders the emergence of critical, well-founded, organized and secure knowledge on their clinical value. Data sharing is a crucial activity which can improve this situation. The paper was written during the preparation phase of SHARE-CTD and affects aspects that are part of the SHARE-CTD subproject being carried out at the LMU (https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-025-03994-3).
First Schooling at Wissenschaftszentrum Schloss Reisensburg
2025.02.01
The 1st schooling of the SHARE-CTD project took place in person with international principal investigators, partners and doctoral students at Wissenschaftszentrum Schloss Reisensburg (Günzburg) from 27.01. to 31.01.2025. The focus was on lectures and exercises around the topics of Reproducibility, Clinical Biostatistics and Clinical Trials, to bring the students with different university backgrounds to the same level. Furthermore, there was a short introduction to the 1st Datathon (07.07.2025 – 11.07.2025, Göttingen, led by Prof. Dr. Ulrich Sax), which ended with the formation of five teams plus homework, such as “Statistical Analysis Plan” and “dynamic reporting”. We were also pleased to have Joseph Ross from YODA (Yale University Open Data Access) joined us via Zoom to present and discuss YODA with our DCs. YODA is an important partner and source for sharing clinical trial data.

In addition to the more serious content and the filling of empty stomachs, there were also social activities for team building, such as a movie night, a pub quiz, poster flash talks and guided tours of “Ulmer Münster” and the museum “Die Einsteins”. According to Albert Einsteins citation “Lernen ist Erfahrung, alles andere ist nur Information” we will meet us again for an exciting 1st Datathon in Göttingen in July 2025!

The winner of our pubquiz night!
Second Schooling event in October 2025 in Lyon
2025.01.29
Led by PhD Evelyne Decullier (Hospices Civils de Lyon) the second SHARE-CTD schooling event will take place at Domaine Lyon Saint Joseph from 19th to 24th of October 2025.
New article Data management and sharing published in JCE
2025.01.23
The SHARE-CTD concept was presented at a session on data sharing at the recent World Congress on Research Integrity (Athens, June 2nd to 5th, 2024, https://www.researchintegrity.org/home-8th-athens-2024) . This session resulted in a topic paper that has now been published in the Journal of Clinical Epidemiology (JCE) PIIS0895435625000137.pdf. The summary of the paper is in line with the concerns of SHARE-CTD: "Responsible data sharing requires strategic planning, cultural shifts in research, and coordinated efforts from all stakeholders to become standard practice in biomedical research".
Panel Discussion Participation
2025.01.09
Martin Posch is going to participate in a panel discussion on IPD meta-analysis organised by ECRIN where also his SHARE-CTD involvement is mentioned in the program.EOSC-ENTRUST Workshop on Support of IPD meta-analyses through TREs-Final Programme and background information_20250107.pdf
Patient centered reanalysis of trials data
2024.12.22
The group of Ulrich Mansmann just published two papers based on clinical trials data sharing: 1) "Multivariable prognostic prediction of efficacy and safety outcomes and response to fingolimod in people with relapsing-remitting multiple sclerosis"article ↗; 2) "Addressing treatment switching in the ALTA-1L trial with g-methods: exploring the impact of model specification" article ↗.
Navigating US participant data sharing requirements: implications for international clinical trials
2024.10.16
An interesting article in BMJ article ↗ we'd like to share with you.
Kick-off meeting and propaedeutic course of SHARE-CTD at Schloss Fürstenried (Munich) from 08.09.2024 to 14.09.2024
2024.09.16
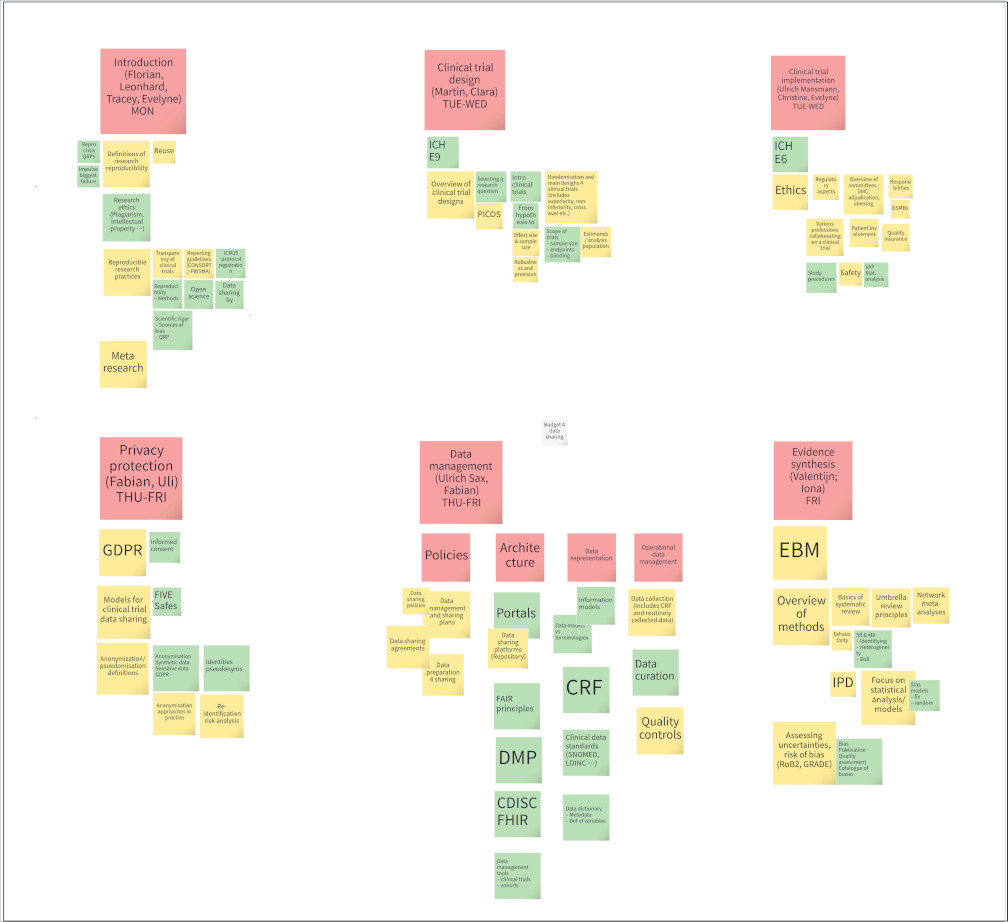
The SHARE-CTD kick-off meeting and the propaedeutic course took place in person with international principal investigators, partners and doctoral students at Schloss Fürstenried (Munich) from 08.09 to 14.09.2024. The focus was on lectures and exercises around the topics Clinical Trial Design, Clinical Trial Implementation, Privacy Protection, Data Management, Evidence Synthesis, to bring the students with different university backgrounds to the same level. To compensate for this rather heavy mental fare, there were games evenings for social networking, a Munich city tour for cultural input and local/ bavarian meals for the physical well-being. The productive and diverse meeting ended with a brainstorming session and some homework for everyone involved. Now, all of us are starting the next three years of the SHARE-CDT project with a lot of hope and energy! And we are looking forward to the 1st schooling event at Reisensburg in January 2025 with all of you!

First Datathon in July 2025 in Göttingen
2024.07.09
Led by Prof. Dr. Ulrich Sax (University Medical Center Göttingen, UMG) the first SHARE-CTD datathon will take place at Rechenzentrum Göttingen from 7th to 11th of July 2025.
SHARE-CTD members met in Munich, at IBE, for a second face-to-face meeting to prepare several topics
2024.06.19
The meeting, in person and via web, was scheduled as a lunch-to-lunch meeting. During Day 1 (17th of June) ideas were collected as building stones for the following three SHARE-CTD training activities: Propaedeutic meeting, first schooling event and first datathon. On day 2 (18th of June), the idea puzzle came together to form a broader picture: a concept to provide our Doctoral Candidates clear and focused learning objectives along the line of Bloom’s Taxonomy: Remember, Understand, Apply, Analyse, Evaluate, Create. The meeting was moderated by Brigitte Strahwald (LMU), an expert in medical education. We are ready for the next steps: to prepare the propaedeutic course in September and to welcome our Doctoral Candidates!
Day 1

Day 2
SHARE-CTD at 8th World Conference on Research Integrity (WCRI) in Athens
2024.06.06
As part of the 8th World Conference on Research Integrity (WCRI) in Athens from 02.06.2024 to 05.06.2024 Prof. Florian Naudet (University of Rennes) will hold a symposium on the following topic: Toward responsible clinical trial data sharing practices. Within this symposium, Prof. Mansmann (Ludwig-Maximilians-University Munich) will also give a lecture on An initiative to train researchers and staff as part of the SHARE-CTD doctoral network. In addition to this lecture, three further lectures on the following topics are planned: (1) Clinical trial data sharing and the global south by Nchangwi Syntia Munung, PhD, Researcher, University of Cape Town; (2) COPE positions about management of empty promises by Daniel Kulp, PhD, senior director (American Chemical Society) COPE (chair); (3) Ethics and incentives for clinical trial data sharing by Jennifer Ellen Miller, PhD, Associate professor, Yale School of Medicine.
Further information on the symposium can be found here: wcri2024.org/symposia
Further information on the 8th WCRI can be found here: wcri2024.org
Paper published in Nature Medicine with participation of SHARE-CTD members
2024.03.25
Members of the SHARE-CTD network coauthored a comment paper published in Nature Medicine (21st of March 2024) on "Understanding the provenance and quality of methods is essential for responsible reuse of FAIR data". nature article ↗
SHARE-CTD members met in Paris for a first face-to-face meeting, after many virtual meetings to prepare the project
2024.02.22

During the two days (February 15 and 16, 2024), we were able to meet on the premises of the French Ministry of Research in the Quartier Latin. We used the time to get a very detailed overview of the planned project activities, to discuss their interlinkages and how our project can benefit from the interaction with ongoing activities of other groups (IRISE, OSIRIS, TIER2). We also met with Christine Kubiac to discuss the role of ECRIN in our project. It was important to run through curricular scenarios in order to bring the planned research work, the concepts to be taught and the active work on data into an effective and synergistic relationship. Most importantly, we did prepare for the active selection phase for the applications received. Thanks to Prof. Dr. Bruno Falissard, a member of our scientific advisory board, for his first contact with us. Special thanks to Florian for organizing this intensive and focused meeting with the support of the Institut Universitaire de France.
The application period is over as of 1st February 2024
2024.02.01
Thanks for all the applications. The named referees can access the application system with their individual links until 07.02.2024. The applicants will be updated about the status of and decisions on their applications in due time.
Kick-off meeting and propaedeutic in September 2024 in Munich
2024.01.15
The Kick-off-meeting will take place in Munich from 08.09. to 14.09.2024 with the Partners, doctoral candidates and guest speakers. The complete program will be announced in time.
Closing date for applications is 31rd January 2024
2024.01.08
Uncompleted applications have to be completed and submitted latest by 31.01.24 in the application portal, after which the system will be available only for named referees until 07.02.2024.
Selection Board established
2024.01.08
For finalizing the recruitment process of the doctoral candidates, the Selection Board formed of all supervisors was established during the Jour Fixe meeting in January 2024.
Implementing clinical trial data sharing requires training a new generation of biomedical researchers
2024.01.08
An interesting nature medicine article ↗ we'd like to share with you.
Making data sharing the norm in medical research
2024.01.08
An interesting nature medicine article ↗ we'd like to share with you.
Data Sharing — A New Era for Research Funded by the U.S. Government
2024.01.08
An interesting article in The NEW ENGLAND JOURNAL of MEDICINE article ↗ we'd like to share with you.

